The Succeeding Patent Edge: Strategic Implications for Pharma Revenue, Portfolios and Innovation
This report from Towards Healthcare, a sister firm of Precedence Research, provides a comprehensive analysis of the upcoming pharmaceutical patent expirations, highlighting their strategic impact on revenue, portfolios and innovation.
Ottawa, Jan. 12, 2026 (GLOBE NEWSWIRE) -- More specifically, the pharmaceutical industry is on the peak for several substantial revolutions. Where numerous products are on the way to lose their patent and foster the changes, which pose risks, as well as immersive opportunities.
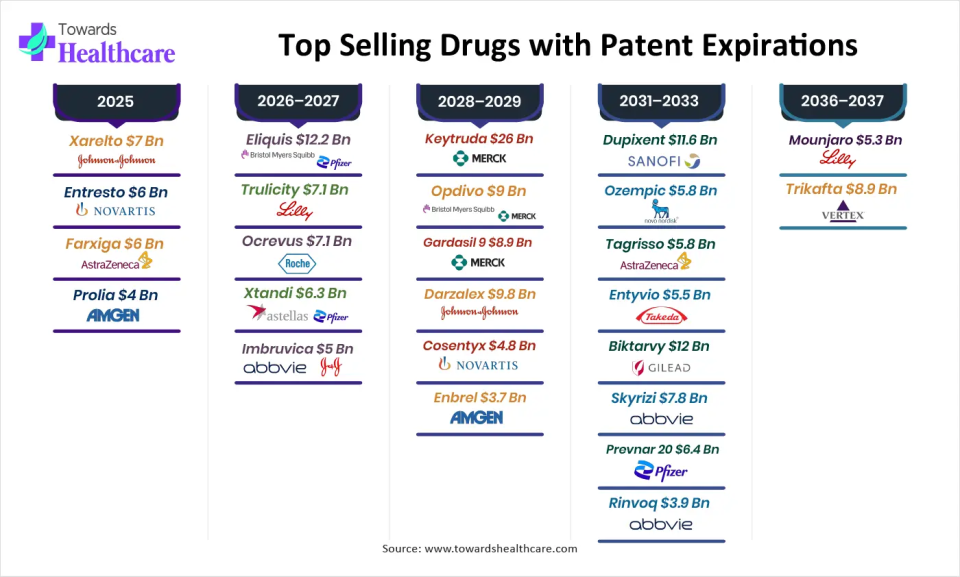
Quick Table:
| Year | Drug | Revenue (USD) | Company |
| 2025 | Xarelto | $7 Bn | Johnson & Johnson |
| Entresto | $6 Bn | Novartis | |
| Farxiga | $6 Bn | AstraZeneca | |
| Prolia | $4 Bn | Amgen | |
| 2026–2027 | Eliquis | $12.2 Bn | Bristol Myers Squibb / Pfizer |
| Trulicity | $7.1 Bn | Eli Lilly and Company | |
| Ocrevus | $7.1 Bn | Roche | |
| Xtandi | $6.3 Bn | Astellas Pharma / Pfizer | |
| Imbruvica | $5 Bn | AbbVie / Johnson & Johnson | |
| 2028–2029 | Keytruda | $26 Bn | Merck |
| Opdivo | $9 Bn | Bristol Myers Squibb | |
| Gardasil 9 | $8.9 Bn | Merck | |
| Darzalex | $9.8 Bn | Johnson & Johnson | |
| Cosentyx | $4.8 Bn | Novartis | |
| Enbrel | $3.7 Bn | Amgen | |
| 2031–2033 | Dupixent | $11.6 Bn | Sanofi / Regeneron |
| Ozempic | $18.4 Bn | Novo Nordisk | |
| Tagrisso | $5.8 Bn | AstraZeneca | |
| Entyvio | $5.5 Bn | Takeda | |
| Biktarvy | $12 Bn | Gilead Sciences | |
| Skyrizi | $7.8 Bn | AbbVie | |
| Prevnar 20 | $6.4 Bn | Pfizer | |
| Rinvoq | $3.9 Bn | AbbVie | |
| 2036–2037 | Mounjaro | $5.3 Bn | Eli Lilly |
| Trikafta | $8.9 Bn | Vertex Pharmaceuticals |
Giant Pharma Firms & Their Expired Patent Revenue
2025-2026
- In recent days of 2026, Xarelto (rivaroxaban), a key anticoagulant with $7 annual revenue, co-developed by Johnson & Johnson and Bayer, was further marketed with a notable price reduction strategy, with an exact patent expiration in 2024.
- Novartis made top selling of Entresto, with its nearly $6B annual revenue, and its first generic versions were introduced in July 2025 by MSN Pharmaceuticals via Novadoz and Torrent Pharmaceuticals.
- With an approximate $6B annual sales performance in 2023-2024, AstraZeneca developed Farxiga to treat Type 2 diabetes (T2D) in adults and children aged 10 years and older.
- In 2025, Amgen's Prolia (denosumab) lost its core protection, with over $4B annual revenue, for the extensive use in osteoporosis and bone-loss treatment.
2026-2027
- Substantial sales of Eliquis (apixaban) in 2023 were of $12.2B, under the crucial role of the alliance of Bristol Myers Squibb and Pfizer. This will further move towards the U.S. patent's expiration, which is set to be between 2026 and 2027. And, European markets will have patient expiration during the second half of 2026.
- Around 2026, AbbVie and Johnson & Johnson will lose patent for Imbruvica, while Amgen, Mylan, Biocon, etc., are developing to launch biosimilar or generics for the same.
- In 2027, Eli Lilly's Trulicity (dulaglutide) will face the U.S. patent expiration, and had $7.1B annual revenue.
2028-2029
- Around 2028-2029, Ocrevus will lose patent, while Roche is focusing on emerging novel formulations and delivery methods to distinguish it from upcoming competition.
- Astellas Pharma & Pfizer’s Xtandi has different expiration years for patent, such as in the U.S., it is 2027, in Europe, it will lose its patent in 2028, and in Japan, it will expire in 2029. Also, they are bolstering research steps in broadening the drug's label to newer patient populations & encourage innovative formulations to extend patent protection.
- Keytruda is set to expire in 2028, and Europe has extended until 2030-2031. However, Merck established a vast "patent wall" around Keytruda, which encompasses approximately 300 patent applications that cover various indications and formulations
- BMS & its partner are stepping into the patent expiration of Opdivo, which is predicted in the US around 2028-2029 and in the EU around 2026. Also, they are preferring efforts in early-stage cancers, adjuvant settings, and combination therapies with other agents.
- Merck is involved in a strategy to perform two large-scale clinical trials in males and females aged 16-26 to find the safety and effectiveness of a single-dose regimen for Gardasil 9, with patent expiration in 2028.
- Darzalex is set to lose its patent protection in the U.S. and Japan in 2029, with Europe around 2031-2032. The company focused on Phase 2 of a combination of Darzalex Faspro with teclistamab, which enhances progression-free survival and overall survival for relapsed/refractory multiple myeloma.
- Novartis unveiled the DTP platform on November 1, 2025, which offers Cosentyx at a 55% discount off the list price, and the product will expire in 2029 & 2030 in the U.S. & European exclusivity, respectively.
- The final U.S. patent expiration of Enbrel is in 2028 and 2029, which will begin the U.S. market to biosimilar competition.
Explore structured pharmaceutical market insights focused on long-term value creation, lifecycle strategy, and post-exclusivity dynamics @ https://www.towardshealthcare.com/download-sample/5341
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

